Develop medical device CERs in compliance with MDR, MDCG and MEDDEV guidelines, as well as clinical reports for other worldwide markets.
Clinical Data Requirements and Evaluation
The MDR has raised the bar on clinical evidence requirements compared to the MDD, including increased evidentiary requirements for legacy devices and new restrictions on leveraging equivalence. In addition, there is increased scrutiny of clinical evidence by multiple regulatory bodies involved in the MDR conformity assessment process. This can present a number of key challenges to manufacturers:
-
Clinical Evaluation Plans (CEPs) and Clinical Evaluation Reports (CERs) are required for all devices regardless of risk class and require frequent updates
-
CERs are labor intensive requiring specialized cross-functional expertise
-
Planning, collection and analysis of clinical data, and the development of compliant CERs is a time-consuming and complex process which often takes weeks or months to complete, and can take years to finalize if data collection is required.
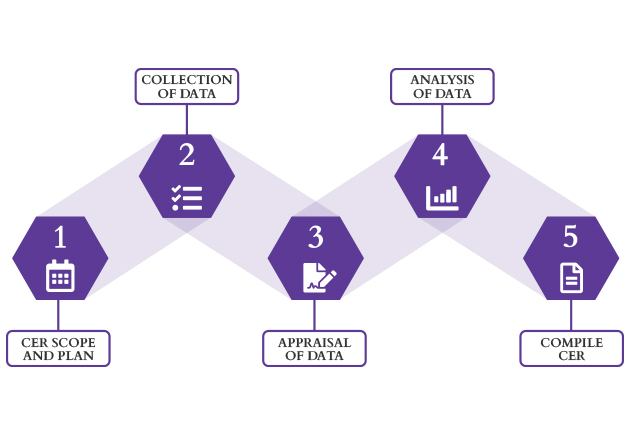
Clinical evaluation is a systematic and multi-stage process:
-
Procedures – MCRA can create compliant procedures related to the development of the clinical evaluation plan (CEP), clinical development plan (CDP), clinical evaluation report (CER), summary of safety and clinical performance (SSCP), post market clinical follow-up (PMCF), conducting literature reviews, and data appraisal methodology
-
Planning – MCRA can perform an analysis of available data to determine the sufficiency of clinical evidence supporting the subject device indications and claims; this includes an assessment of any pre-market investigation data, post-market data, and/or equivalent comparator data and determines the need for pre-market or specific post-market clinical activities
-
Preparation – MCRA can develop the entire CEP, CDP, CER, SSCP, PMCF Plan and PMS Plan or specific sections per the established guidance including equivalence justifications, clinical investigation summary, literature review (see below), post-market data assessment and summary, and benefit-risk assessment
-
Analysis - MCRA can perform comprehensive literature reviews including database searches and safety and performance analyses to determine the state-of-the-art in the clinical indication and key safety and performance metrics
-
Review – MCRA can assist manufacturers in a review capacity to ensure drafted documents are sufficient and meet all regulatory requirements.
-
Evaluation – As part of the CER process, MCRA will utilize all data sources to conduct a benefit:risk assessment to determine the benefit:risk profile of the device to demonstrate the device conforms with the relevant GSPRs or other regulatory requirements.
-
Integration – MCRA can ensure integration and alignment of the clinical evidence, claims, benefits and risks throughout the technical documents, ensuring risk management, clinical evaluation and accompanying documentation are fully integrated.
CER Process
Our Experienced Team
-
MCRAs global regulatory team has years of experience in the planning, execution, and integration of the clinical evidence process, including developing new clinical documents or updating existing documents
-
Our team of trained regulatory specialists with an understanding of regulatory writing and subject matter knowledge can ensure integration of your technical documents with clinical evidence
-
Capability to conduct comprehensive state-of-the-art literature reviews and analysis across multiple databases with multiple reviewers
-
Integration of Clinical, Regulatory, & Quality experts who understand the content of a successful clinical evidence generation, evaluation, and review process
-
MCRA’s team can function independently or as an extension of your own team with a clear focus on successful and rapid market entry
Contact a Regulatory Affairs Expert
