The EU and UK are both under a period of legislative change for In Vitro Diagnostic (IVD) medical devices.
By: Erica Conway, Vice President, IVD Regulatory Affairs - Europe
The UK has recently published an Amendment to its Medical Devices Regulation *[1] (UK MDR 2002), which is the Statutory Instrument related to the transition time that an EU CE marked device may be used to access the UK market *[2].
The change in UK legislation for medical devices following the UK leaving the EU, and the transition in the EU of the EU IVD Directive (IVDD) to IVD Regulation (IVDR), taken together creates challenges for Manufacturers. It is important how these changes work together in order to prepare a suitable strategy and path forward, where the CE mark is being used to access the Great British (GB) market *[3].
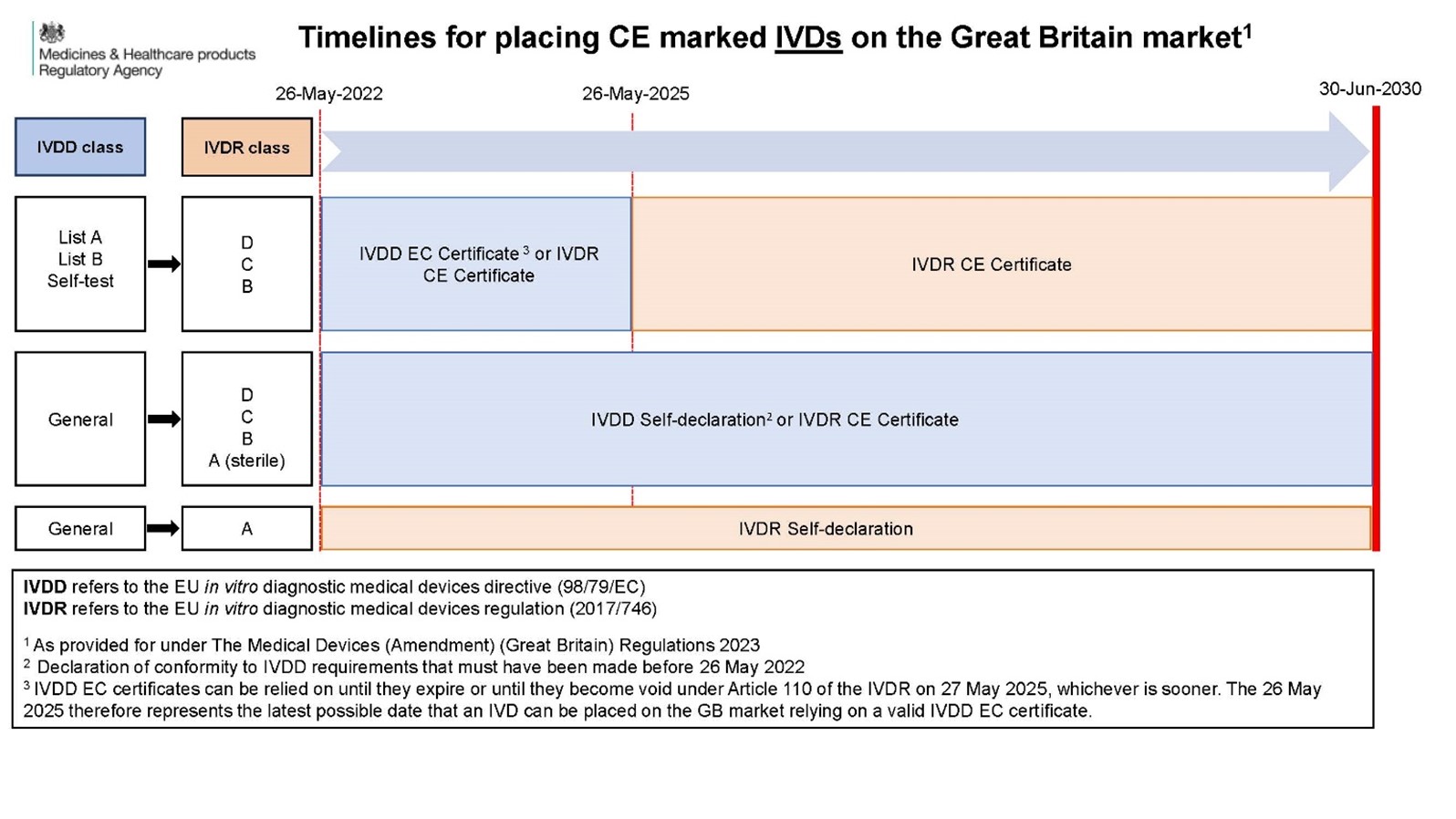
The MHRA has recently published an infographic to support with the understanding of the UK MDR 2002 amendment *[4].
What do these transition timelines mean for a Manufacturer of IVDs that are destined for the UK market?
‘General IVDs’ under what was IVDD, are now up-classified under IVDR:
-
For devices self-declaring to the EU IVDD (with a signed Declaration of Conformity, DoC, by 26 May 2022), using this DoC for GB market access is only possible for as long as the IVDR Article 110 provisions allow. Therefore, manufacturers should also refer to IVDR Article 110 for the relevant deadlines for using this DoC for the GB market.
-
If the device is certified to the IVDR, it is possible to use this IVDR CE mark until 30 Jun 2030 for GB market access.
-
The MHRA infographic only concerns using CE-marking to access the GB market. It is worth remembering that ‘UKCA marking’ is possible now. If you have a ‘general’ IVD under UK MDR 2002, it is possible to ‘self-declare’ against the UK MDR 2002 until 2030, if the DoC is signed prior to the new UK MDR 2002 effective date (expected 1 July 2025). After 1 July 2025 we expect the new UK legislation to apply (as it stands currently) for new devices, but the previously signed DoC can still be relied on up until 1 Jun 2030 – this is our current understanding!
IVDs that needed a Notified Body (NB) for CE marking under IVDD:

-
Certificates issued by an EU Notified Body (NB) under the IVDD (including HIV tests, blood glucose meters, and Self-Test devices) will have an expiry date that will be either 26 May 2025, or sooner! ‘Renewal’ of IVDD certificates is not possible. These devices will need to be certified to the IVDR before their EC certificate expiry date. Therefore, the same will be true for GB market access if the CE mark is being used.
-
If the device is certified to the IVDR, it is possible to use this IVDR CE mark until 30 Jun 2030 for GB market access.
-
It is possible to apply for a 'UKCA mark' for these devices now (against current UK MDR 2002) from an IVD Approved Body (AB). It is understood that this certificate will go beyond the 26 May 2025. We are waiting to hear how quickly a manufacturer would need to transition from the old to new UK MDR 2002, following the effective date of the new UK legislation for these devices.
Devices that were self-declared under the IVDD and are still self-declared under IVDR (i.e. Class A non-sterile):

-
Class A devices that are non-sterile, e.g. instruments, should be IVDR compliant from 26 May 2022. The self-declaration should be against the IVDR for those devices being placed on the market after 26 May 2022. This CE-mark can be used for GB market access until 30 Jun 2030.
-
It is possible for manufacturers to self-certify against the UK MDR 2002. This will allow continued GB market access. Manufacturers should be aware of any new provisions that take effect when the new UK MDR 2002 is published.
What can Manufacturers do now?
In order to maintain access to both the EU and UK (GB) markets requires a clear strategy and planning by the Manufacturer. The transition from the EU IVDD to the EU IVDR, in addition the changes to legislation in the UK market mean a plan should be made, if not created already, from now to the relevant deadlines, to then plan out the regulatory changes and practical impacts that need to be worked through by all sectors within a company.
If you need support to understand and work on your project planning for compliance and maintaining devices on the EU and UK markets, contact the team at MCRA (info@MCRA.com).
Reference: (from https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1166483/Infographic_-_Devices_transition_timeline.pdf)
[1] The Medical Devices Regulations 2002, UK Statutory Instruments 2002 No. 618, https://www.legislation.gov.uk/uksi/2002/618; also referred to as UK MDR 2002
[2] UK MDR 2002 Amendment: https://www.legislation.gov.uk/uksi/2023/627
[3] Note that Northern Ireland, due to the Northern Ireland Protocol, must follow the requirements of the EU IVDR. CE CE marking will continue to be needed for devices placed on the Northern Ireland market and EU rules apply.
[4] MHRA infographic for CE marking transition: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1166483/Infographic_-_Devices_transition_timeline.pdf